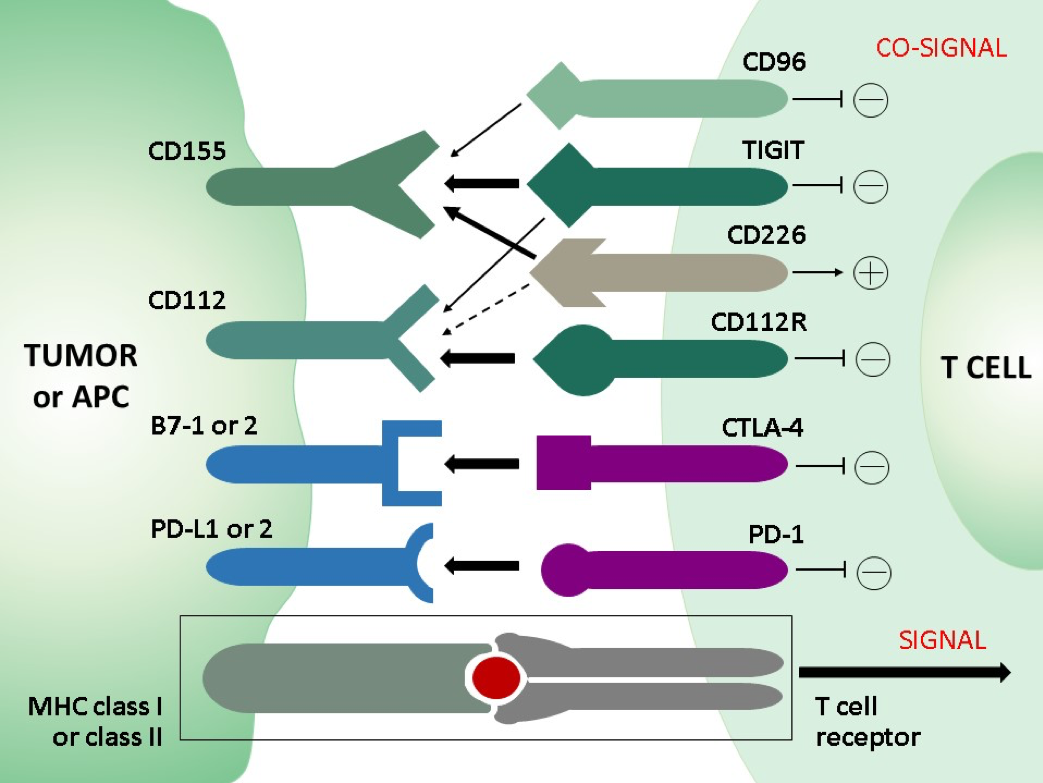
In normal immune homeostasis co-inhibitory and co-stimulatory receptors on T cells negatively and positively regulate the TCR stimulation in concerted action thereby fine tuning the T cell activation in order to limit unwanted self-tissue damage. Whereas in acute infection CD8 effector T cells turn into memory cells after clearance of the pathogen, a persistent antigenic stimulation and inflammation either with nonself antigens in chronic infection or with self-antigens in the tumor microenvironment (TEM) in cancer leads to a progressive loss of function in CD8 T cells, referred to as “T cell exhaustion”. A phenotypic hallmark of exhausted CD8 T cells is the upregulation and sustained expression of multiple and different combinations of inhibitory receptors (immune checkpoints), including PD-1, CTLA-4, TIM-3, LAG-3, and others, such as TIGIT or the recently discovered novel immune checkpoint CD112R. This implies that inhibitory receptors co-regulate T cell exhaustion. The involvement of inhibitory receptors in T cell dysfunction led to the development of new therapeutic strategies aiming at blocking the inhibitory receptors with monoclonal antibodies (checkpoint blockade) in order to restore the T cell function. The outstanding clinical success of therapies with checkpoint inhibitors against PD-1, PD-L1 or CTLA-4 in terms of overall survival of cancer patients proves the therapeutic potential of immune checkpoints and gives rise to new promising targets and combinatorial approaches for immune checkpoint blockade strategies. Monoclonal antibodies against TIGIT did not only block its inhibitory effect but also shifted the balance in favor to the co-activating receptor CD226 which shares the TIGIT ligands. TIGIT and CD266 form a pathway that is analogous to that of the co-inhibitory receptor CTLA-4 and the co-activating receptor CD28.