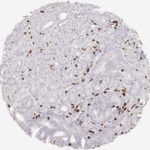
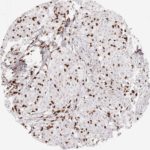
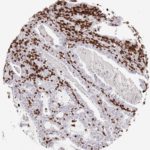
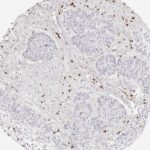
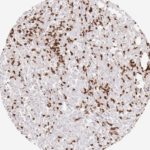
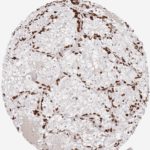
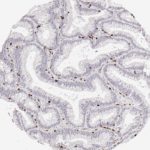
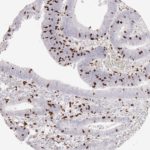
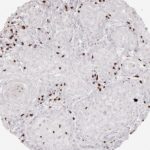
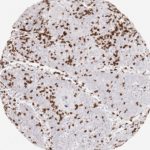
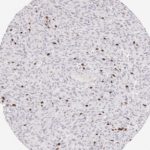
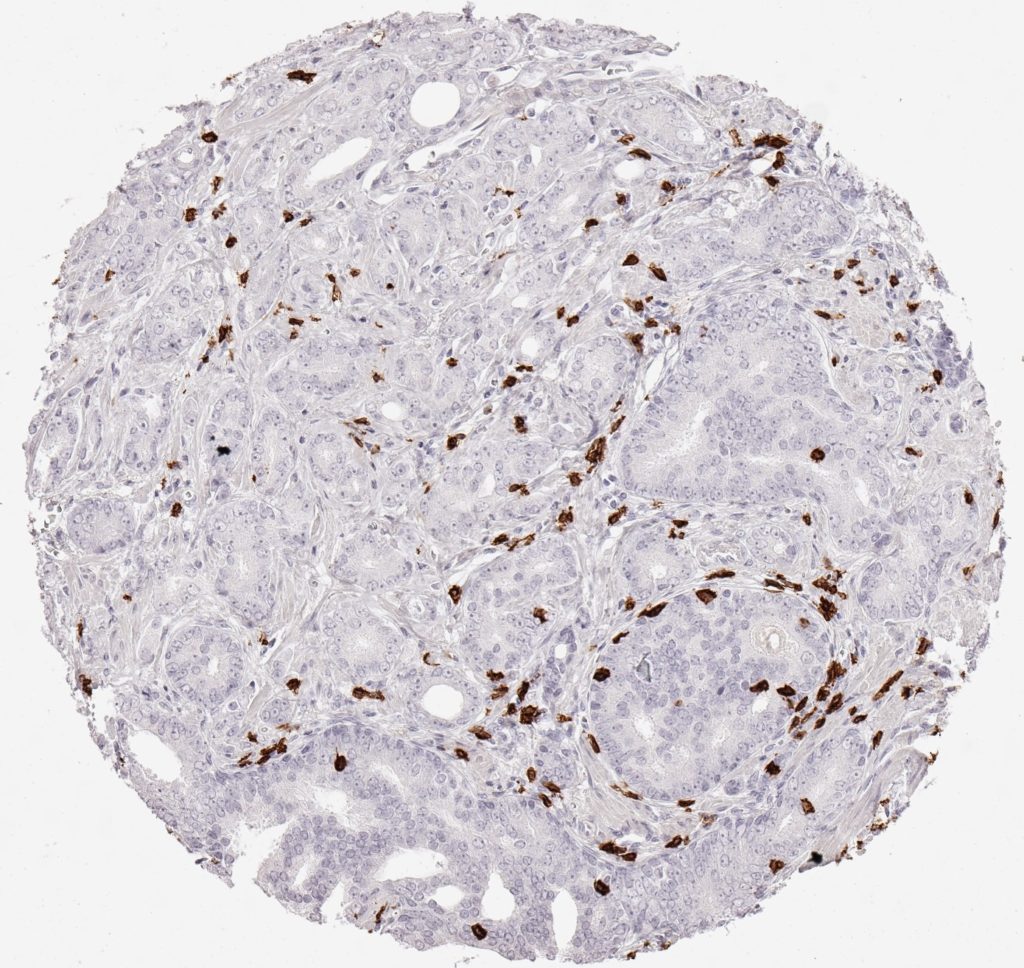
Der CD8-Antikörper Klon TC8 wurde speziell für den immunhistochemischen (IHC) Nachweis von CD8 in menschlichen FFPE-Routine-Gewebeproben entwickelt. Klon TC8 wurde für einen optimalen Signal-Rausch-Kontrast optimiert und für die Identifizierung von CD8+-tumorinfiltrierenden T-Zellen (TILs) validiert, mit dem Ziel, einen unübertroffenen spezifischen Nachweis von CD8 in der Tumormikroumgebung zu ermöglichen.
CD8+ T-Zellen spielen eine zentrale Rolle bei der Vernichtung von Krebszellen. Sie haben die Fähigkeit, verschiedene menschliche Tumore zu infiltrieren und sind an der Entwicklung eines spezifischen Tumormikroumfelds beteiligt. Krebszellen haben Mechanismen entwickelt, um der Immunreaktion gegen den Tumor erfolgreich auszuweichen, indem sie hemmende Signale durch Hochregulierung der Expression immunsuppressiver Komponenten erzeugen. Eine wirksame Blockade dieser Interaktion wird als ein wichtiger Faktor bei der Entwicklung von Krebsimmuntherapien angesehen. Darüber hinaus scheinen bereits vorhandene CD8+ T-Zellen die Wirksamkeit solcher Immun-Checkpoint-Therapien vorherzusagen.
Der T-Zell-Rezeptor (TCR) erkennt spezifische antigene Peptide auf der Oberfläche von Krebs- und anderen Zielzellen, die von HLA-I/β2m-Komplexen präsentiert werden. Die Bindung an den TCR induziert eine Signaltransduktionskaskade, die zur Ausführung zytotoxischer T-Lymphozyten-Funktionen (CTL) führt. Während CD8+ T-Zellen direkt an antitumoralen zytotoxischen Reaktionen beteiligt sind, ist die Beteiligung von CD4+ T-Zellen eher indirekt, z.B. durch ihre Hilfe beim Priming von CD8+ T-Zellen.
Im Gegensatz dazu werden inhibitorische T-Zell-Rezeptoren wie PD-1, CTLA-4 und TIGIT durch die immunsuppressive Tumormikroumgebung mit dem Ziel aktiviert, tumorinfiltrierende Lymphozyten (TILs) zu inaktivieren. Zu den derzeit wirksamsten Krebsimmuntherapien gehören die Immun-Checkpoint-Inhibition ICI und die Blockierung von T-Zell-inhibierenden Rezeptoren. Darüber hinaus scheint eine wirksame Blockade-Immuntherapie mit dem Vorkommen von CD8+ T-Zellen assoziiert zu werden.
Specifische Referenzen für Klon TC8
1.
Blessin et al. Patterns of CD112R expression in normal lymphatic tissues, inflammation and cancer. Proceedings: AACR Annual Meeting 2020; April 27-28, 2020 and June 22-24, 2020; Philadelphia, PA, Volume 80, Issue 16 Supplement, pp. 3870.
DOI https://doi.org/10.1158/1538-7445.AM2020-3870
2.
Simon et al. Prognostic role of CD112R, PD-1 and Ki67 expression in CD8+cytotoxic T cells in colorectal cancer. Proceedings: AACR Annual Meeting 2020; April 27-28, 2020 and June 22-24, 2020; Philadelphia, PA, Volume 80, Issue 16 Supplement, pp. 4970.
DOI https://doi.org/10.1158/1538-7445.AM2020-4970
3.
Eichenauer, T. et al. High level of EZH2 expression is linked to high density of CD8-positive T-lymphocytes and an aggressive phenotype in renal cell carcinoma. World J Urol (2020).
DOI https://doi.org/10.1007/s00345-020-03200-4
4.
Fraune, C. et al. MMR Deficiency is Homogeneous in Pancreatic Carcinoma and Associated with High Density of Cd8-Positive Lymphocytes. Ann Surg Oncol 27, 3997–4006 (2020).
DOI https://doi.org/10.1245/s10434-020-08209-y
5.
Blessin et al. Prevalence of CD8+ cytotoxic lymphocytes in human neoplasms. Cellular Oncology 2020, 43: 421-430.
DOI https://doi.org/10.1007/s13402-020-00496-7