Reactivity
Clone TG1 is the first monoclonal antibody detecting TIGIT in routine formalin-fixed paraffin-embedded (FFPE) tissues. TG1 has been validated for the detection of TIGIT positive T-cells with a focus on tumor infiltrating T cells (TILs). Anti-TIGIT clone TG1 allows the identification of TIGIT in the tumor microenvironment under pathological conditions.
TIGIT (T-cell immunoreceptor with Ig and ITIM domains) is a member of the poliovirus receptor (PVR) family. TIGIT is expressed on subsets of T lymphocytes and controls an immune checkpoint. The expression of TIGIT has been reported on NK cells, regulatory T cells, follicular T helper cells, memory CD4+ T cells, and CD8+ T cells, but TIGIT is not expressed on B cells or naive CD4+ T cells. Naive CD4+ T cells may upregulate TIGIT expression upon activation. In many different cancer models, TIGIT has been shown to be upregulated on T cells. The ligands CD155 and CD112 are also highly expressed on dendritic cells and macrophages in different cancer diseases. Moreover, TIGIT expression is highly correlated with the expression of further co-inhibitory molecules like PD-1. In addition to its direct inhibitory effect on cytotoxic T-cells, TIGIT can stimulate an immunosuppressive microenvironment by influencing other immune cells: For example, TIGIT can bind CD155 on the surface of dendritic cells or is able to manipulate NK cell activity. TIGIT inhibiting drugs are currently being developed. Immunohistochemical (IHC) application of monoclonal antibody TG1 may provide valuable information for clinical research and potential therapeutic interventions specifically targeting TIGIT at its cancer immunology checkpoint.
IHC protocols
Staining protocols for anti-human TIGIT antibody clone TG1
| Cat.No.: |
DIA-TG1 (200µg) |
| Concentration: |
0.4mg/ml |
| Isotype: |
Mouse IgG1/k |
| Specificity: |
Human TIGIT |
| Physical State: |
Lyophilized powder |
| Reconstitution: |
DIA-TG1 (200µg), restore to 500µl. Reconstitute with sterile destilled water by gentle shaking for 10 minutes. |
| Technical Note: |
Antibody clone TG1 stains cell membranes of various lymphocyte subtypes. Weak non-specific nuclear/nucleolar staining may occur in some epithelial tissues (i.e. colon cancer). |
Procedure 1 Manual Immunostaining
IHC FFPE tissue
Slide preparation
-
mount 4µm sections on superfrost slides
-
deparaffinize tissue sections in xylene (2x 5min)
-
rehydrate in descending ethanol series (100%, 90%, 80%, 70%)
-
rinse 5 minutes in TBST* buffer
Pretreatment (epitope retrieval)
- autoclave for 5 min** at 121°C in TEC*** buffer pH7.8 (1x concentration)
- rinse 5 minutes in TBST* buffer
Peroxidase-blocking
- incubate 10 min in Peroxidase-Blocking Solution (#S2023, DAKO REALTM)
- rinse 2x 5 min in TBST* buffer
Antibody incubation
- dilute primary antibody (TG-1, stock 1.966 mg/ml) 1:50 – 1:150 in antibody diluent (#S2022, DAKO REALTM)
- cover tissue section with 100-200µl diluted antibody
- incubate 1h at 37°C in moist chamber
- rinse 2x 5 min in TBST* buffer
- apply DAKO EnVision Polymer-HRP mouse/rabbit Kit (#K5007, DAKO REALTM) according to manufacturer’s recommendation
- rinse 2x 5 min in TBST* buffer
Chromogen
- cover slides for 10 min with DAB-Chromogen (DAKO EnVision Polymer-HRP mouse/rabbit Kit, #K5007, DAKO REALTM)
- wash slides in H2O dest. thoroughly
- counterstain for 20 sec mit Hematoxylin (Mayer’s Hematoxylin 41-5131-00, Medite GmbH)
- develop for 5 min in water
- dehydrate in ascending ethanol series
- wash in xylene
- apply mounting medium and coverslips
* TBST wash buffer (#K8000, DAKO)
** Incubate 5 min after autoclave has reached 121°C
*** TEC:
Stock solution = 20x Tris-EDTA-citrate buffer: per 1 liter aqua dest. dissolve 5g Trizma base (Sigma T 1503), 10g EDTA (Merck 1.08418.0250), 6.4g tri sodium citrate (Sigma C 0909), adjust to pH 7.8 using HCL 1mol.
Figures
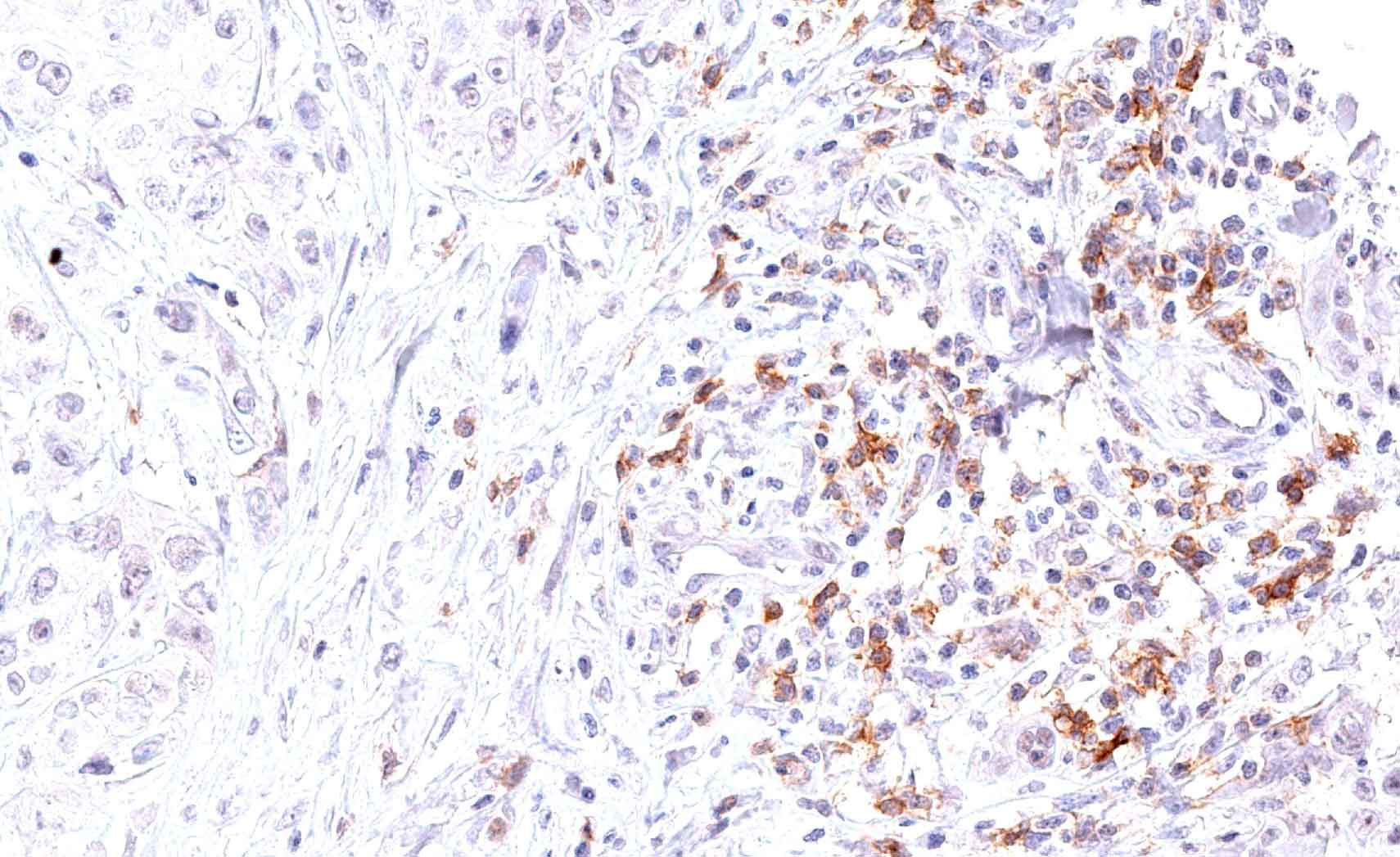
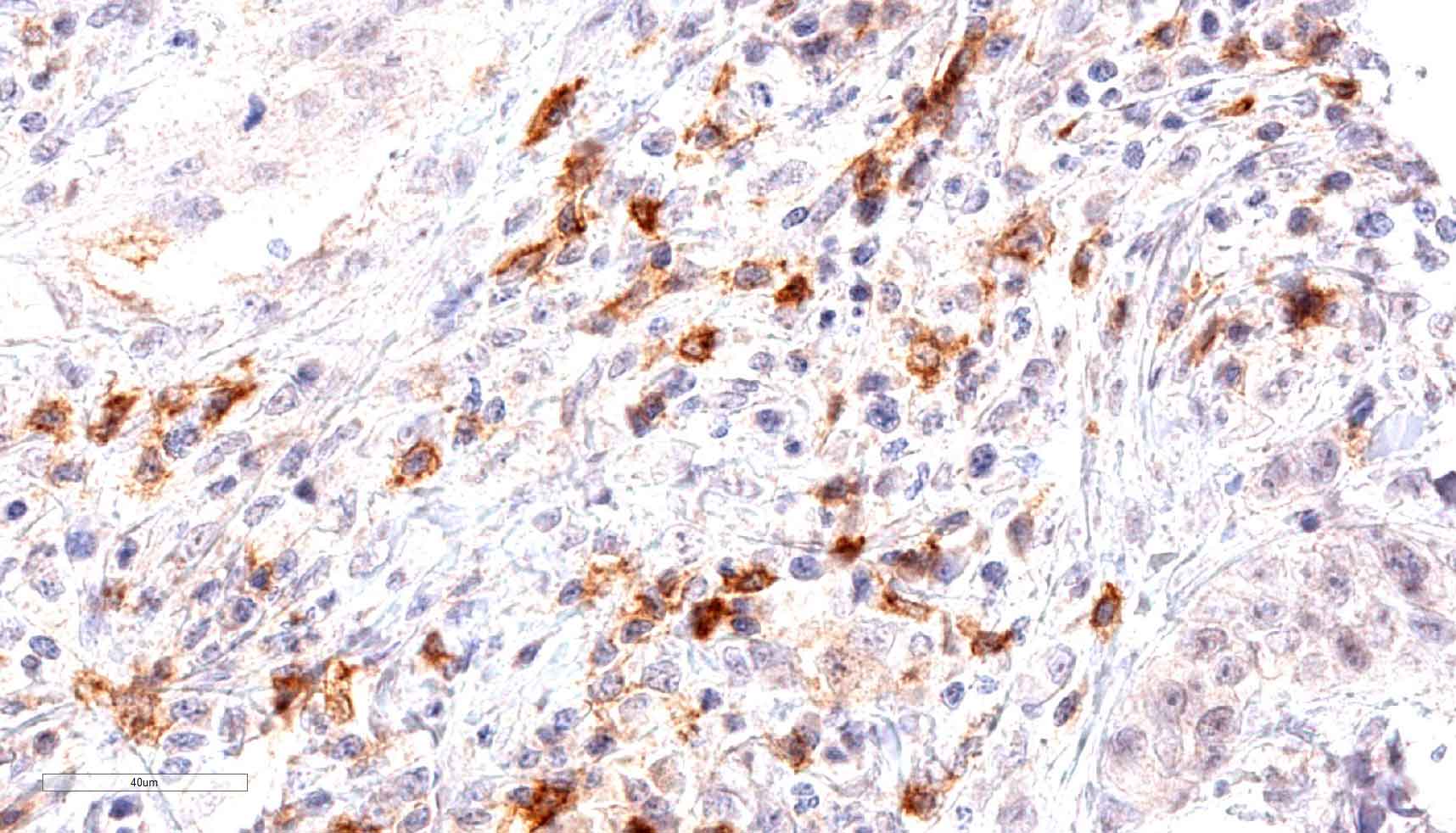
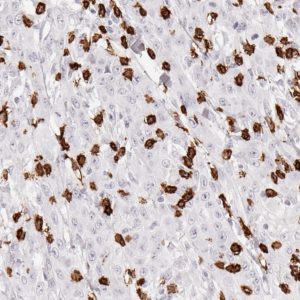
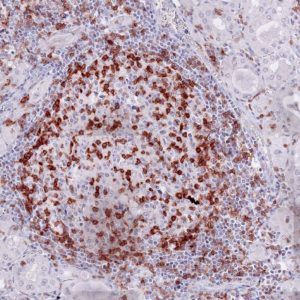
Fig.1:
Microphotographs of dianova‘s TG-1 immunostaining on human tonsil
Antibody dilution 1:50
Antigen retrieval:
Autoclave in TEC*-buffer (pH7.8) at 121°C for 5 min (*Tris-EDTA-Citrate)
Images taken with Zeiss Axiocam MRc camera and AxioVision SE64 software, fitted to a Zeiss Axioskope 40 microscope (200x). No image enhancement or manipulation.
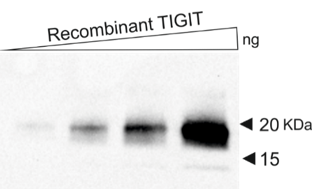
Fig. 2:
Western blot using recombinant TIGIT protein as a target for dianova TG-1
Procedure 2 Automated Immunostaining
Leica Bond RX
- Dewax & rehydrate sections
- Heat pretreatment before entering the slides into the system HIER.l (heat induced epitope retrieval) High pH buffer, pH 9.0, 100°C, 20min. (even better: Autoclave at 121°C, 5min)
- Cool slides
- Rinse with Bond wash solution 3x
- Peroxide block: Leica Microsystems, 5min.
- Rinse with Bond wash solution 3x
- Primary antibody: anti-TIGIT / clone TG1, 1:50, 37°C, 60min.
- Rinse with Bond wash solution 3x
- Post Primary Leica Microsystems, 30min.
- Rinse with Bond wash solution 3x 2min.
- Polymer Leica Microsystems, 30min.
- Rinse with Bond wash solution 2x 2min
- Rinse with deionized water 1x
- Substrat-Chromogen: Mixed DAB Refine, 10min.
- Rinse with deionized water 3x
- Counterstain: Hematoxylin Leica Microsystems, 5min.
- Rinse with deionized water 1x
- Rinse with Bond wash solution 1x
- Mount
References
Publications clone TG1
1.
Li W et al. Expression of the immune checkpoint receptor TIGIT in Hodgkin’s lymphoma. BMC Cancer 2018, 18: 1209.
doi.org/10.1186/s12885-018-5111-1
2.
Blessin NC et al. Patterns of TIGIT expression in normal lymphatic tissue, inflammation and cancer. Disease Markers 2019, Jan 10;2019:5160565. eCollection 2019.
doi.org/10.1155/2019/5160565
3.
Hinsch A et al. et al. Expression of the immune checkpoint receptor TIGIT in seminoma. Oncol Lett. 2019, 18: 1497–1502.
doi: 10.3892/ol.2019.10428
4.
Scimeca, M. et al. Programmed death ligand 1 expression in prostate cancer cells is associated with deep changes of the tumor inflammatory infiltrate composition. Urol. Oncol. 2019, 37, 297.e19-297.e31.
doi: 10.1016/j.urolonc.2019.02.013
AACR Annual Meeting / Chicago / Apr 14-18, 2018
Poster Presentations:
Patterns of TIGIT expression in normal lymphatic tissue, inflammation and cancer
Department of Pathology, University Medical Center Hamburg – Eppendorf, Hamburg, Germany
Session Title: Inflammation, Immunity, and Cancer
Apr 15, 2018, McCormick Place South, Exhibit Hall A, Poster Section 32
download poster
High variability of TIGIT expression in Hodgkin’s lymphoma
Department of Pathology, University Medical Center Hamburg – Eppendorf, Hamburg, Germany
Session Title: The Metastatic Microenvironment
Apr 16, 2018, McCormick Place South, Exhibit Hall A, Poster Section 6
download poster
The expression and significance of PVR-TIGIT/CD226, an immune checkpoint axis in small cell lung cancer
Hirsch Biomarker Analysis Laboratory, Anschutz Medical Campus, University of Colorado Denver, Aurora, CO, USA
Session Title: Immune Checkpoints 3
Apr 17, 2018, McCormick Place South, Exhibit Hall A, Poster Section 26
download poster